Using Insulin Pens for Insulin Delivery
Welcome to our Insulin Pen Overview and Frequently Asked Questions (FAQs) page. As health care providers, we know that insulin pens are widely used for insulin delivery and while they are relatively simple to use, many have questions about their usage and best practices. In this section, we address some common queries related to insulin pens, including their functioning, selection, storage, disposal and more.
What Are the Types of Insulin Pens?
Pens come as either disposable prefilled pens or reusable pens loaded with a cartridge that you can fill with insulin from a vial. Many of the reusable pens are considered “Smart Pens” because they are connected to an app that provides data such as a dose and time stamp log and reminder options, or connectivity to a glucometer or continuous glucose monitor (CGM).
The disposable pens are prefilled with insulin and available from a pharmacy in the pre-filled state. The reusable pens are either filled by the user with pre-filled cartridges of insulin or by adding insulin from a vial to a compatible cartridge.
As previously mentioned, connected insulin pens have additional features for management. These include the ability to log dose, food, events and activity, and glucose values. They allow for data interpretation, the ability to set reminders, connect to glucometers and continuous glucose monitors, and use of the data for dosing recommendations.
How Does One Choose the Correct Pen?
The decision on which insulin pen to prescribe is influenced by the providers recommendation for insulin type, insurance coverage (formulary), and whether a connected pen (Smart Pen) or conventional pen is the best option for them.
To see all pens and insulin delivery devices available in the U.S., view our find and compare insulin delivery devices page.
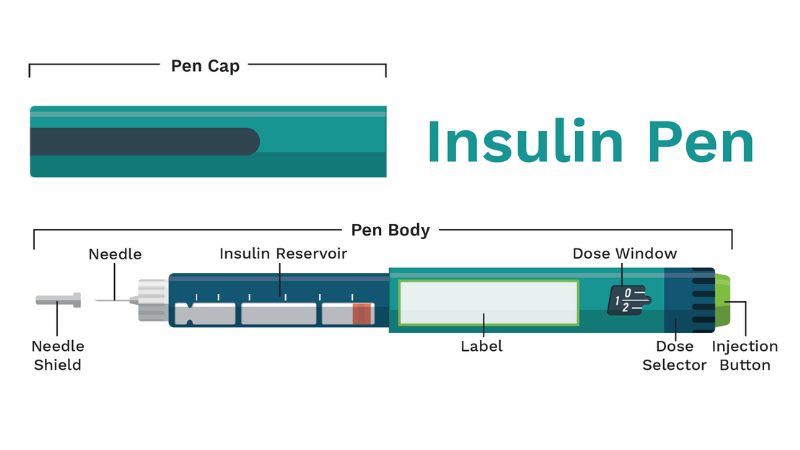
What are the typical parts of an insulin pen?
![]()
Insulin pens come in different types, but they all have the similar basic components. It's important for insulin pen users to understand how each of these elements work together to ensure that the medication is administered correctly.
- One important component is the pen cap, which helps to protect the insulin when the pen isn't in use
- Another component is the rubber seal, which connects the pen needle to the body of the pen.
- The insulin reservoir is where the insulin is stored, and there is a clear plastic area that allows you to see how much insulin is left in the pen.
- Additionally, there is a label that indicates the type of insulin and the expiration date,
- The dosage selector is used for dialing the correct dose
- A dose window displays the number of insulin units selected
- Finally, an injection button is pressed down to give the injection.
How do I properly use an insulin pen, and what are some tips for injecting insulin with a pen?
Fore detailed instructions, read Learning How to Inject which includes choosing a device, injection techniques, and site rotation charts.
Additional tips include:
- Insulin pens are intended for use by one person and should not be shared.
- It is critical to rotate sites around the body and within an appropriately large area of each site to help keep the skin tissue healthy.
- Before injecting a mixed insulin dose, gently roll the pen between the palms to mix the contents and do not shake.
- Prime the pen before each use to remove air bubbles (directions in Learning How to Inject).
- Count to 10 after the insulin pen button has been fully depressed before pulling the needle out of the injection site.
- Only use a needle pen once and dispose of it properly (see below).
- Dial up the dose and confirm it again before administering the injection rather than going on autopilot.
- Tips to ensure the correct pen is used:
- Insulin pens are clearly labeled with the medication name and reusable pens are color coded.
- Consider adding further labeling with a permanent marker or homemade label to ensure that no mix-ups occur.
- Some people put a rubber band on their long-acting insulin pen, so they don’t confuse it with their short acting pen.
- Check the label with every injection.
Can insulin pens be reused, or do I need to dispose of them after one use?
Insulin pens are available as both reusable and disposable. Disposable pens require a prescription for the insulin as a pen option not a vial. Reusable pens require a prescription for the reusable pen and also require a prescription for the prefilled insulin cartridge (the user needs one prescription for the reusable pen and one for the insulin and refills). The cartridges or disposable pens are disposed of when they are empty or after 28-32 days (after opening depending on the insulin).
How do I store my insulin pen, and how long can I use it before it expires?
Store unused insulin pens, insulin filled cartridges, or insulin in a vial in the refrigerator in the refrigerator door. This makes it easier to find and prevents it from being pushed to the back of the refrigerator where it may be at risk of freezing. Insulin will have an expiration date on it, encourage the person with diabetes to check this date before they leave the pharmacy or upon receiving it by mail. An awareness of expiration dates and using their insulin supplies in order of their expiration will help individuals avoid wasting insulin because it expired. Things do happen and sometimes insulin does expire- talking about that upfront will prevent the person with diabetes from blaming themselves unnecessarily. If this happens often, a log book may be helpful for supply management.
Insulin pens can be kept at room temperature for varying amounts of time. Make sure that the user is aware how long insulin can be kept out at room temperature for their prescribed insulin.
Most disposable insulin pens and insulin cartridges need to be discarded 28-32 days after opening. This is dependent on the insulin and it is recommended that you go over this information during their insulin pen education.
Are insulin pens covered by insurance, and how much do they cost?
Insulin pens and the insulin that they hold are covered by insurance. There is a co-pay cap of $35.00/insulin for individuals with private insurance and individuals on Medicare who get insulin through their prescription benefit. (Capping the cost of insulin at $35.00 under Medicare Part B coverage will take effect July 1, 2023.)
Can I travel with my insulin pen, and are there any special precautions I need to take?
You can and will need to travel with your insulin pens, additional insulin, and supplies. It is advised to pack insulin in your carry-on bag instead of your checked luggage to avoid damage from changes in air pressure or temperature extremes in the luggage compartment of an airplane. When the person with diabetes is traveling by plane, provide them with a copy of their prescription for all medication as well as any extra supplies that they will be carrying with them that requires a prescription. This helps them get through airport security easier (hopefully) and facilitates getting supplies if they need them while they are traveling.
According to the FDA insulin may be left unrefrigerated at a temperature between 59°F and 86°F for up to 28 days. Cooling packs designed for keeping medications cold but not frozen, or a refrigerated cooling device may be helpful if temperatures are going to be questionable. Remind the user to not use insulin that has been frozen, to keep insulin out of direct heat or light and to avoid exposing insulin to extreme temperatures.
A TSA Disability Card is helpful to facilitate an easier travel experience.
Medication that is over 3.4 fluid ounces is exempt from the TSA prohibitions as is juice, gel, glucagon, etc. to treat hypoglycemia.
You are allowed to request that TSA hand checks your diabetes supplies and does not send it through the X-ray machine. While Insulin can withstand the X-ray machine, you are permitted to request hand checking of your medication and supplies.
How often should I change the needle on my insulin pen, and how do I properly dispose of used needles?
The recommendation is to use a new needle for each injection or to change it at least 1x/day. Dispose of used needles in a sharps container. Repurposing a clean empty laundry detergent bottle for disposal works for some people based on the local sharps disposal ordinances. See guidelines for your locale here.
NEVER throw sharps “loosely” into regular garbage- an injury may occur when disposing of items that are not handled properly.
What are some common side effects of using an insulin pen, and when should I contact my health care provider?
It is a good idea to check injection technique, discuss site rotation, and periodically observe the person giving an injection if possible. Assure the person with diabetes that all questions about giving themselves an injection are valid and anything that they notice out of the ordinary should be brought to your attention. Since there are no nerve endings in the layer of fat below the skin, the injection should not be very painful. Advise the person with diabetes, that if the injection is causing pain, that they should let you know about it.
Common side effects of using an insulin pen include:
Lipohypertrophy: Lipohypertrophy is a collection of fatty tissue that forms under the skin that occurs due to repeated injections in the same place. It is commonplace in people with diabetes, can impact your body's ability to absorb insulin and has the potential to cause serious complications. One way to decrease the likelihood of lipohypertrophy is to practice site rotation that uses different parts of the body and rotates within the same sight in a large area. This resource provides guidance and charts for educating on and logging site rotation locations.
Hypoglycemia: A person who uses insulin to manage their diabetes is at risk of hypoglycemia. Understanding the symptoms of hypoglycemia and how to treat a hypoglycemic event are an integral part of education around using insulin. Everyone who has a prescription for insulin should also have a prescription for glucagon and be educated in how to use it and how to educate those around them in case of an emergency where they themselves cannot act.
Redness, swelling or itching at the injection site may also occur as a result of injecting insulin. Guide those with diabetes to report any of these side effects to you as soon as they are able.
How do I troubleshoot common problems with my insulin pen, such as leaks or malfunctions?
- Prime your pen according to the directions before each injection to avoid injecting air and to prevent the buildup of air bubbles in the pen that can lead to a malfunction.
- To avoid leaking, always store your pen without the needle attached.
- When attaching a new needle, hold the pen in an upright position pointing the tip into the air. Tap the side of the insulin pen to move any air to the top of the fluid to be ejected in the priming shot.
- Never depress the plunger without a needle attached to prevent the build-up of pressure.
- If you notice any cracks in the outside of the pen or the plunger mechanism or the dose counter is not moving properly, discontinue use of that pen and return it to the pharmacy for a replacement.
- Always count to 10 when injecting after the pen has been depressed before removing from the skin.
Is there any way to lessen the pain of the injection?
- Change the needle frequently to avoid dull needles.
- Use an appropriate size (length and thickness) needle. It is advised to use the shortest, thinnest needle available. Needles that are too long may lead to painful intra-muscular injections with faster insulin absorption.
- Make sure that you inject into the fat pad and not the muscle. Choose the right length needle for the amount of fat on the body. Use of a shorter needle will help prevent injecting into the muscle below the fat pad that is found right below the layer of skin. The recommendation is smaller is better both for pain reduction, proper injection technique into the fat pad, and for consistent insulin absorption. If there is very little fat, you may need to pinch the fat pad and inject into that as shown in this resource Insulin Injection Know-How.
- Inject at a 90-degree angle or at the appropriate angle for the specific needle size used and body fat pad thickness.
- Rotate sites and keep track of site rotation using a system like the one in this resource.
How do I know that I got the right dosage?
- It is important to keep the pen in place for a count of 10 once you inject the insulin to ensure that the complete dose was delivered.
- Avoid injecting into any areas damaged by lipohypertrophy. Injecting into these areas will lead to poor insulin absorption.
- Remember to inject into the fat pad and not the muscle.
Any tips to ensure I don’t get my long acting and short acting mixed up?
Insulin pens are clearly labeled with the medication name and reusable pens are color coded. Recommending that a person with diabetes who uses more than one insulin adds further labeling with a permanent market or home-made label may help to ensure that no mix-ups occur. In addition, encouraging them to check the label every time they inject and not function on autopilot will further help avoid mix-ups.
How much insulin is typically in a pen?
Most pens hold 3 ML (300 units of insulin for U100, 600 units of insulin for U200, 900 units of insulin for U300, etc.).
Do they always need to be refrigerated and if not, how long do they last in normal temperatures?
Insulin may be left unrefrigerated at a temperature between 59°F and 86°F for up to 28 days.
What causes the insulin in pens to “go bad” or be less effective other than age?
Keep insulin out of direct heat or light and avoid exposing it to extreme temperatures (hot or cold) to help ensure effectiveness.
Do all needles fit all pens?
Most pens can be used with various needles. It is important to help the person with diabetes choose the most appropriate needle size for them based on needle length and diameter in order to only inject into the fat pad sitting directly underneath the skin. Listen to this episode of The Huddle podcast for more information.
Do all dose in full units or are there pens that dose in smaller half doses for children or those sensitive to insulin?
There are pens that dose in half unit doses. These currently include the Humalog Junior Quick Pen and the reusable InPen and NovoPen Echo.
As this may change, you can search for this on danatech by selecting “Half Unit Dose Capability” on the Medicine Delivery page.
Bleeding occurred when I injected, did I get the full dose?
Sometimes a drop of blood will leak out after the injection, have the user press the area lightly with clean gauze or a tissue. As long as they held the pen in for the entire dose and counted to 10 after the dose was administered, they will have gotten the entire dose. If they see a fluid leaking out and it smells like insulin, have them contact the office, so you can have them monitor their glucose levels for safety.
Related danatech Resources


 Guidance on clinical assessment, adjustments, report interpretation and key education. Designed to be used during clinic visits.
Guidance on clinical assessment, adjustments, report interpretation and key education. Designed to be used during clinic visits.-(3).jpg?sfvrsn=16486d59_5)